What is the Educational Dose Illustrator?
What is the Educational Dose Illustrator?
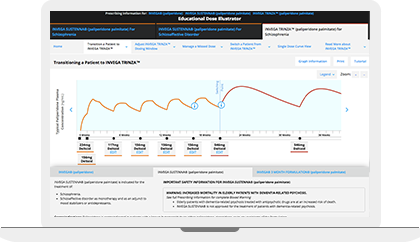
The Educational Dose Illustrator can be used to visualize how dosing affects paliperidone plasma concentrations following administration of INVEGA SUSTENNA® and INVEGA TRINZA®. This resource simulates the paliperidone plasma concentrations over time resulting from different dosing scenarios that are set forth in the respective Prescribing Information for INVEGA SUSTENNA® and INVEGA TRINZA®.
What is the Educational Dose Illustrator?
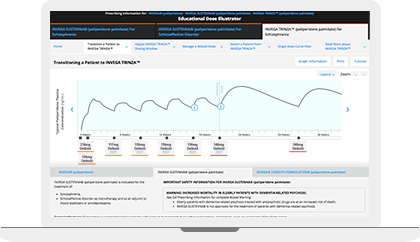
The Educational Dose Illustrator can be used to visualize how dosing affects paliperidone plasma concentrations following administration of INVEGA SUSTENNA® and INVEGA TRINZA®. This resource simulates the paliperidone plasma concentrations over time resulting from different dosing scenarios that are set forth in the respective Prescribing Information for INVEGA SUSTENNA® and INVEGA TRINZA®.
Line Colors
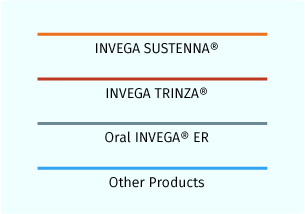
Various line colors represent different products. A red line is used to represent INVEGA TRINZA® and an orange line is used to represent INVEGA SUSTENNA® plasma concentrations. Additional line colors are utilized in the switching scenarios for other products.
Editing
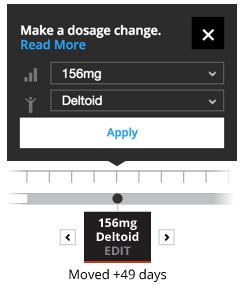
The Educational Dose Illustrator allows you to change the timing, injection location, or dosage strength when permitted within the illustrative scenario.
Prior to Transitioning a Patient to INVEGA TRINZA®, Establish a Consistent INVEGA SUSTENNA® Maintenance Dose
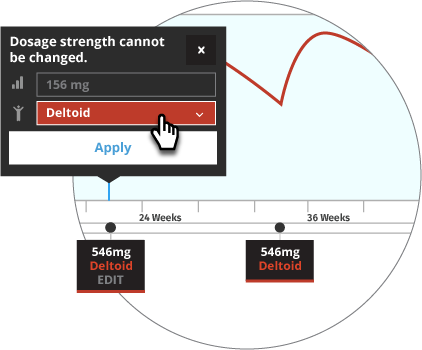
The recommended initiation of INVEGA SUSTENNA® is with a dose of 234 mg on treatment Day 1 and 156 mg one week later, both administered in the deltoid muscle. INVEGA TRINZA® is to be used only after INVEGA SUSTENNA® has been established as adequate treatment for at least four months. The last two maintenance doses of INVEGA SUSTENNA® must be the same dosage strength before starting INVEGA TRINZA®.
For illustrative purposes only, the INVEGA SUSTENNA® site of injection (deltoid or gluteal muscle) is based upon the first maintenance dose selection, and maintenance doses may only be adjusted one dosage strength higher or lower from the prior INVEGA SUSTENNA® injection. In most of the scenarios, an additional INVEGA SUSTENNA® maintenance dose is also displayed (as either at least four one-month injections or two initiation doses and three one month injections) for illustrative purposes only.
Prior to Transitioning a Patient to INVEGA TRINZA®, Establish a Consistent INVEGA SUSTENNA® Maintenance Dose
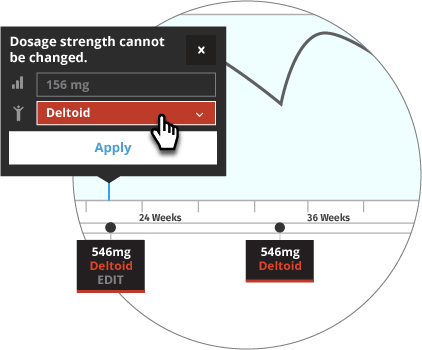
The recommended initiation of INVEGA SUSTENNA® is with a dose of 234 mg on treatment Day 1 and 156 mg one week later, both administered in the deltoid muscle. The last two maintenance doses of INVEGA SUSTENNA® must be the same dosage strength before starting INVEGA TRINZA®. INVEGA TRINZA® is to be used only after INVEGA SUSTENNA® has been established as adequate treatment for at least four months.
For illustrative purposes only, the INVEGA SUSTENNA® site of injection (deltoid or gluteal muscle) is based upon the first maintenance dose selection, and maintenance doses may only be adjusted one dosage strength higher or lower from the prior INVEGA SUSTENNA® injection. In most of the scenarios, an additional INVEGA SUSTENNA® maintenance dose is also displayed (as either at least four one-month injections or two initiation doses and three one month injections) for illustrative purposes only.
Transitioning a Patient to INVEGA TRINZA®
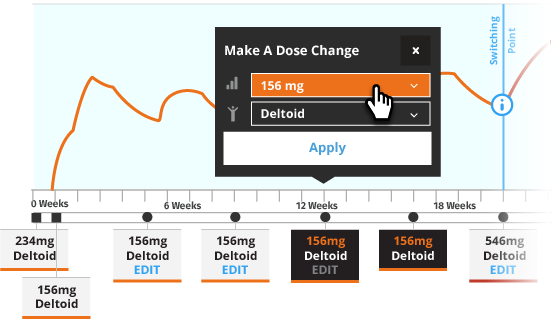
Initiate INVEGA TRINZA® when the next INVEGA SUSTENNA® dose is scheduled with a 3.5-fold higher dose in either the deltoid or gluteal muscle. Following the initial INVEGA TRINZA® dose, INVEGA TRINZA® should be administered every 3 months in either the deltoid or gluteal muscle. INVEGA TRINZA® dose adjustment can be made every 3 months in increments within the range of 273 mg to 819 mg based on individual patient tolerability and/or efficacy.
For illustrative purposes only, all INVEGA TRINZA® maintenance dosage strengths are locked as the dose is based on a 3.5-fold higher dose of the previous INVEGA SUSTENNA® dose. The site of injection for INVEGA TRINZA® is based upon the site of injection selected for the first INVEGA TRINZA® dose. Additionally, in most illustrative scenarios, the timing of the INVEGA TRINZA® dose is locked and cannot be adjusted ±2 weeks to help avoid a missed dose.
Transitioning a Patient to INVEGA TRINZA®
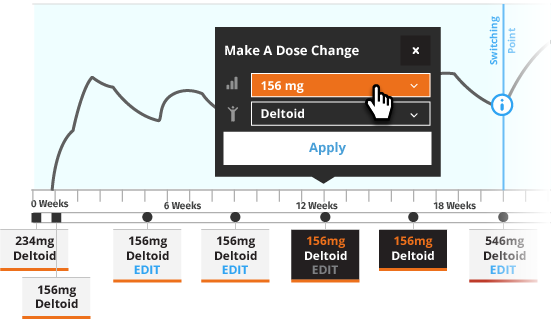
Initiate INVEGA TRINZA® when the next INVEGA SUSTENNA® dose is scheduled with a 3.5-fold higher dose in either the deltoid or gluteal muscle. Following the initial INVEGA TRINZA® dose, INVEGA TRINZA® should be administered every 3 months in either the deltoid or gluteal muscle. INVEGA TRINZA® dose adjustment can be made every 3 months in increments within the range of 273 mg to 819 mg based on individual patient tolerability and/or efficacy.
For illustrative purposes only, all INVEGA TRINZA® maintenance dosage strengths are locked as the dose is based on a 3.5-fold higher dose of the previous INVEGA SUSTENNA® dose. The site of injection for INVEGA TRINZA® is based upon the site of injection selected for the first INVEGA TRINZA® dose. Additionally, in most illustrative scenarios, the timing of the INVEGA TRINZA® dose is locked and cannot be adjusted ±2 weeks to help avoid a missed dose.